Got a feeling you’re not alone? Forget alien life forms—we’re talking an altogether different kind of critter: the trillions of microbes that call your body home. The majority of these tiny creatures live in your gastrointestinal tract. But how did they get there? What do they do? What happens when they get out of whack? And what can we do about it?
Meet your microbiome
WHAT ARE MICROBES?
Trillions of tiny organisms—microbes—are all over, and inside, each of our bodies. They’re on our skin and genitals, and in our mouths, ears and sinuses. By far the majority of them, though, camp out in our gut. Estimates are that there are between 30 trillion and 400 trillion microorganisms in the human gut, and from three to 100 times more bacteria in the gut than there are cells in the human body.
When we talk about microbes hanging out in the ‘gut’, what we mean is the gastrointestinal tract—the series of long, hollow organs that run in a long, twisting tube from the mouth to the anus—including the oesophagus, stomach, small intestine and large intestine. Because few microbes can survive the acidic environment of the stomach for long amounts of time, the further you go down the gastrointestinal tract, the more microbes you’ll find.
But what exactly is a microbe? Microbes, also known as microorganisms, are living creatures that can only be seen under a microscope. Some have just one cell, while others are multicellular. Their small size means that, individually, microbes have almost no impact on us. However, vast populations of microbes acting together are capable of profound effects.
Let’s zoom in and take a closer look at the kinds of microbes in our gut.
First of all, it’s useful to know that scientists group all life on Earth into three main groups called domains. The three domains are Eukaryota, Archaea and Bacteria, with microbes being found in each. Each of these domains can then be grouped further into more specific categories (think of them like the branches of a tree) called phyla. Each phylum is divided up into more and more specific categories (smaller and smaller branches), until we eventually reach the various species.
Bacteria are single-celled microorganisms with some distinctive cell structures. They make up around 90 per cent of our gut’s population (the remainder includes Fungi and Archaea). The collective name for all the kinds of microorganisms that typically live in the gut is the gut microbiota. Because we have such a special, lifelong relationship with our gut microbes, the collective name we give to those in any one person’s gut is their gut microbiome.
The kinds of bacteria in our gastrointestinal tract fall into five main phyla: Bacteroidetes, Firmicutes, Actinobacteria, Proteobacteria and Verrucomicrobia. Of these, Bacteroidetes and Firmicutes are usually the most common. Within each of these phyla, there are a variety of species of bacteria—more than 1,000 altogether. Proteobacteria, for example, includes Escherichia coli (some strains of which are pathogenic) and Salmonella.
The proportions of each of these species in our gut vary widely between individuals. Since bacteria act in numbers, the relative proportions of species (community structure) fundamentally impact how our microbiome works. But until very recently this critical aspect of our gut ecosystem has been impossible to measure. A terrestrial ecologist wanting to describe a plant community can readily sample more than 50 per cent of the trees in even a large environment. By contrast, a microbial ecologist wanting to sample 0.001 per cent of the bacterial community of one person’s gut by culture-based techniques would need to get around one billion bacterial colonies and use around 100,000 tonnes of agar. Annual global production of agar is only 10,000 tonnes!
It is only in the past 10 years that it has become possible for microbial ecologists to contemplate a description of the microbiota in any habitat. Now, new technologies such as genetic sequencing machines can rapidly describe microbial communities in terms of genetic markers, bypassing the need to culture. A recent study, for instance, used computational analysis to look at how many and what kinds of microbes are in the gut and found that bacterial species are even more diverse than previously thought.
HOW DID THEY GET THERE?
As a baby in the womb, we’re in a bacteria-free environment, protected by our mother’s body and immune system from the world outside. But this is the last time we’ll be able to call every cell in our bodies our own.
As soon as the amniotic sac is broken, we are exposed to bacteria. If we are born via a vaginal delivery, we begin by getting a dose of microbes from the birth canal, but if born by caesarean section our first dose is from the skin of our carers. Once we emerge fully into the world, we’re quickly exposed to myriad bacteria—from skin and breastmilk, and from microbes in the place of birth, such as the hospital. In just a few days, these ‘pioneering immigrants’ outnumber our own cells. Differences due to this initial exposure can be seen in the microbial community of babies for several weeks at least, although the significance of this is hotly debated.
These early days, months and years of our lives are a time of flux for our microbes—it’s a changing, unstable system. One study, looking at the first six months of life, revealed a great diversity in microbial populations between babies, with a surprisingly wide variation in what constitutes a ‘healthy’ microbiota. As we grow, and begin to put all kinds of things into our mouths (not just a variety of foods, but all those other goodies that toddlers like to taste test) we introduce new microbes into our gut.
Sometime between a period of six months and three years, a stable microbiome composition is attained. This happens in association with the adoption of an adult-like diet and the completion of immune development. By the time we’re adults, our gut microbes have calmed down somewhat, with each person’s microbial communities having become more similar to each another. They remain stable for periods of a year or more (in the absence of certain external factors, discussed below) in terms of the types of species present, though varying in the abundance of the different species.
As we move through life, our microbiota is affected by disease states; changes in age, diet, and geographical location; intake of food supplements and drugs, including antibiotics; and other environmental influences. So it’s perhaps not surprising that there are still differences in the microbial make-up of individual adults—our own gut microbiota is unique to each of us. There are also differences between populations.
WHAT DO THEY DO?
While it used to be thought that bacteria are bad, we now know that’s not the whole story. Some, such as Salmonella, are harmful to us. But many are harmless, and others, it seems, are positively helpful. Complicating the picture further, some can be both ‘bad’ and ‘good’. Helicobacter pylori, for example, can trigger the formation of gastrointestinal ulcers but has recently been found to have benefits for appetite regulation.
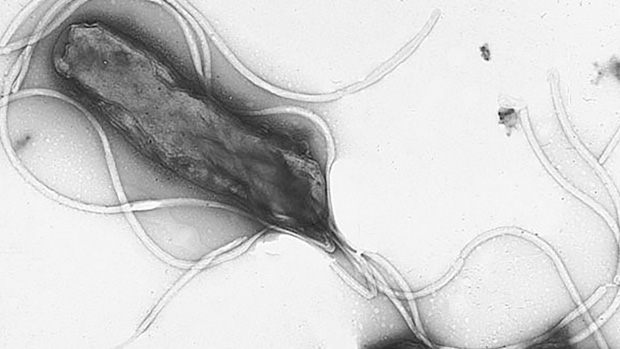
If we take a step back and consider our microbiome as a system, we can see that microbes and people live in a mutually beneficial (symbiotic) relationship. We feed them goodies and, in return, they do a whole heap of things for us, like:
- break down foods
- supply the gut with energy
- make vitamins
- break down toxins
- protect against pathogens, (by setting up camp where the harmful bacteria normally would, and by producing anti-microbial chemicals that defend the host against them).
As well as all this, our gut microbes may also have an important role in ‘training’ our immune system. As it encounters a diversity of microbes in the gut, the immune system learns which (pathogenic) bacteria to attack and which (beneficial) bacteria to leave alone. The development of defined arms of the immune system thus goes hand in hand with the acquisition of a complex microbiota.
When your gut gets out of whack
In high-income countries, we’re increasingly seeing alterations in the composition and function of the gut microbiota. Numerous potential explanations have been put forward, including extensive use of antibiotics, which can decimate microbe populations; changes in what we eat (especially the trend towards more highly processed foods); and more hygienic living conditions, which have led to the decline of some potentially useful microbes in the gut. Whatever the causes are, there is no doubt that we have changed our association with microbes, and the consequences are turning out to be numerous. Let’s take a look at some of them.
OBESITY
People whose intestines have smaller and less diverse bacterial populations appear to be more prone to obesity.
Comparing lean and overweight people in a study of twins, researchers found that the gut community in lean people was very diverse. They had a wider variety of Bacteroidetes, for instance, which specialize in breaking down bulky plant starches and fibers into shorter molecules that the body can use as a source of energy.
In another experiment, by researchers at Washington University in St Louis, Missouri, germ-free mice were populated with bacteria from lean and obese women. Even though they were fed the same diet and in the same amounts, those with the bacteria from the obese women grew fatter (also associated with a less diverse gut microbiota). Next, the lean mice were placed in the same living quarters as the obese mice. The obese mice were found to have taken on board some of the lean mice’s bacteria—probably through eating their poo. Consequently, the obese mice became leaner.

The absence of certain microbes may also mean we may not be able to effectively regulate our appetites, potentially leading to obesity. Martin Blaser of New York University found that the microbe H. pylori helps regulate appetite by modulating levels of ghrelin—a hunger-stimulating hormone. Although it was once common in our digestive tracts, the prevalence of H. pylori in many countries is now lower, thanks to more hygienic living conditions and the use of antibiotics.
ANXIETY AND DEPRESSION
Some studies have revealed a connection between gut microbiota and the brain. In experiments in mice, for example, changes in gut microbial composition were found to change the animal’s brain chemistry, making them either bolder or more timid.
More specifically, gut microbes may have a role to play in anxiety and depression. For example, people with gastrointestinal disorders such as Crohn’s disease, ulcerative colitis and irritable bowel syndrome have been found to suffer from mood disorders like anxiety and depression that can’t just be explained as a reaction to being unwell.
We’ve seen how microbes make things our bodies need, such as vitamins. They also produce hundreds of neurochemicals needed by the brain, including those ‘feel good’ types, dopamine and serotonin, among others. At present, however, there is no conclusive evidence that microbially produced serotonin makes it from the gut to the brain.
Despite a lot of interest in the link between the gut and the brain, and, by extension, the possibility of finding new ways to treat mood disorders, research in this area is still in its infancy; most studies have been carried out on rodent, rather than human, subjects.

IMMUNE SYSTEM PROBLEMS
Our immune system is a double-edged sword. We need it to combat infections and clear potentially cancerous mutant cells, but an overactive or uncontrolled immune system can do enormous damage. A rise in autoimmune disorders in developed countries has been associated with changes to the microbiota in high-income populations. Since our immune system develops after birth, together with our microbiome, some people have proposed that developed country diets, antibiotic use and the elimination of chronic parasite infections may have resulted in a microbiota that lacks the capacity to establish a balanced immune response, which could account for the increase in autoimmune problems.
Problems in the gut also have the potential to reduce the effectiveness of vaccines, making us more susceptible to epidemic diseases. Many modern vaccines work by introducing a dead or weakened pathogen (including viruses) into the body. This ‘teaches’ the immune system which microbial signals should be considered dangerous and results in the immune system producing protective antibodies, ready to use when a live pathogen enters the body. When the gut is inflamed, however, microbes can ‘leak’ through the gut lining. Busy dealing with them, the immune system is less able to produce antibodies in response to the vaccine. The result? Your immune system potentially won’t have the necessary antibodies to mount a quick attack when a microbe invades for real.
INFLAMMATORY BOWEL DISEASE
Researchers in Japan and at CSIRO have highlighted the important role of microbes in producing a beneficial fatty acid that can help protect against inflammatory disease. Gut microbes degrade some dietary carbohydrates which our body can’t digest, and convert them into short-chain fatty acids such as acetate, propionate and butyrate. These fatty acids, especially butyrate, are an essential component of our nutrition. In experiments on mice, the researchers found that, by switching on genes, butyrate causes the immune system to produce more regulatory T cells, which control excessive inflammatory responses. Conversely, a lack of butyrate has been found in people with inflammatory bowel diseases such as Crohn’s.
Restoring the balance
So our microbiome should be viewed as a part of us and, if we don’t treat it right, it can go wrong—leading to disease. The good news is that it appears that the community structure and activity of microorganisms in the gut can be altered, and fairly rapidly. So, what can we do to keep our microbiota healthy?
GET YOUR FINGERS ON THE PULSE
You’ve probably heard of the benefits of probiotics for the health of our gut microbes—more on them later. But you might also want to consider cracking open a tin of beans or nibbling a not-quite-ripe banana. That’s because one way to encourage a healthy microbiome is to eat more foods containing resistant starch. Carbohydrate that can’t be digested and absorbed in the small intestine is the primary fuel for processes in the colon—and these are dominated by our gut bacteria.
Most starch is digested in the small intestine, with the non-digestible portion travelling on to the large intestine. Here it’s gobbled up by microbes, who release small carbohydrate molecules as they use the starch for energy. The product they excrete is butyrate—the fatty acid we met earlier. It’s used as an energy source by colonocytes and encourages blood to flow into the vessels of the large intestine, keeping it healthy. It even allows the body to more easily detect and destroy cells that have damaged DNA before they can multiply and potentially cause colorectal cancer.
But fatty acids aren’t the only thing these microbes produce during this process—as you’ll know if you’ve ever shared a car with a person who’s been chowing down on chickpea curry. The bacterial breakdown of fibre also generates gas (flatulence)—that’s right, farts.
While feeding our microbes is beneficial, it can be bad news when microbes are starved of fibre. Hungry microbes may start to feed on the protective mucus lining of the gut, which could lead to inflammation and disease.
So, what foods should you be packing in your lunchbox to keep those hungry—and useful—microbes happy? Some foods containing resistant starch include:
- beans and other pulses—their seed coat slows down digestion. Hummus, made from chickpeas, is a great option.
- bananas—eat them a bit green, as most of the starch turns to sugar as they ripen
- potato salad—cooking and cooling potato results in the skin forms a gel structure that resists normal digestion in the stomach
- rolled oats—eat them uncooked for higher levels of resistant starch
- cashews.
Pulses are so important to humans as a food source—providing protein, other essential nutrients and starch—that the United Nations declared 2016 as the International Year of Pulses.

PROBIOTICS
Many of us have seen probiotics on the supermarket shelves, either as capsules or as food products such as yoghurt. But what exactly are they—and do they work?
Probiotics, the most common of which are strains of Lactobacillus and Bifidobacterium, are live organisms. The idea is that, after you’ve swallowed them, they’ll make their way through the stomach and take up residence in your gut.
While some studies have examined the effectiveness of probiotics in humans and mice, it’s still not clear whether they actually make it through the acidic environment of the stomach to colonise the lower intestinal tract—and, if so, how likely it is they will confer a specific benefit once they’re there. Different species of bacteria will have different effects. But it can be difficult, and costly, for producers of probiotic products to prove the specific strains of bacteria which are present in their products are the ones that delivered the changes.
While there is increasingly strong evidence that some types of probiotic bacteria are useful for specific conditions—the best documented are lactose intolerance and rotavirus diarrhoea—more thorough clinical trials are needed for most claims. The jury is still out on probiotics.

POO THERAPY, ANYONE?
In case you don’t like lentils or yoghurt, there are other options being explored for improving the health of the gut and eliminating harmful bugs.
One is faecal (yes, poo) transplants—that is, transplanting healthy faeces from a donor to the colon of a recipient.
We’ve already seen how obese mice became leaner after eating the faeces of lean mice. In another experiment, a diverse microbiota was transferred from a healthy mouse (via its poo) to the gut of a mouse infected with two kinds of antibiotic-resistant bug. Both of these harmful bugs were eliminated in the infected mouse.
Faecal therapy has also been found to be effective in treating patients with the potentially deadly superbug C. difficile, which tends to take hold in people (especially hospital patients) whose community of protective microbes has been decimated by antibiotics. In one clinical trial, the use of faecal transplants was found to be more effective than antibiotic use for eliminating the bug—and even more exciting is that pill-based versions are equally effective. These pills may be thought of as a new style of probiotics in which communities of microbes, rather than one or a few selected strains, are introduced. There are other areas of investigation, too. These include the use of ‘psychobiotics’ —probiotic bacteria to treat mood and anxiety disorders—and microbiome tests to improve the accuracy of bowel cancer screening and the success of weight-loss diets. Compared to poo, perhaps yogurt and lentils don’t look so bad!
Conclusion
Ever since the discovery of microbes (they were first seen in the human mouth), we’ve known that the majority of bacteria that occur on our body are not harmful. However, we didn’t know what they actually do—and the devastating consequences of pathogens have given our microbial buddies a bad rap. Modern research techniques are revealing the many and complex roles of our gut bacteria, and the potential consequences when the crosstalk between the human and microbial cells in our bodies gets out of balance. We are starting to see this knowledge translated in healthcare changes, but we still have a way to go before we fully understand how the microbes in our gut work together, and with the rest of our bodies, as a complex system that keeps us healthy, or makes us sick.










